Self-stirring nanoreactors enhance reaction efficiency for chemical synthesis

Ingrid Fadelli
contributing writer

Gaby Clark
scientific editor

Robert Egan
associate editor

Recent technological advances have opened new possibilities for the efficient and sustainable synthesis of various valuable chemicals. Some of these advances rely on nanotechnologies, systems or techniques designed to design and study materials or devices at the nanometer scale.
Nanoreactors are nanotechnologies designed to host and control chemical reactions within confined spaces. These small structures serve as tiny "reaction vessels" that enable the precise manipulation of the reactants, catalysts and conditions to elicit desired chemical reactions.
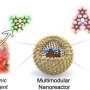
Researchers at Inner Mongolia University, Fudan University and Julin University in China recently developed a new paddle-like mesoporous silica nanoreactor that can stir itself when exposed to a rotating magnetic field. This nanoreactor, outlined in a paper in Nature ��������, can mix chemicals at a molecular level, enhancing the efficiency of reactions and thus potentially enhancing the synthesis of various compounds.
"Developing artificial nanomaterial systems that can convert external stimuli to achieve nanoscale self-sustainable motion (for example, self-rotation), and simultaneously integrate and deploy the spatial localization of multiple active sites to unravel the intraparticle diffusion patterns of molecules, is of great importance for green synthetic chemistry," wrote Yuzhu Ma, Peiting Guo and their colleagues in their paper.
"We show a paddle-like self-stirring mesoporous silica nanoreactor system with separated chambers and controllable proximity of active sites."
The new nanoreactor developed by Ma, Guo and their collaborators is essentially a tiny, sponge-like structure with pores through which molecules can pass through. Notably, when a rotating magnetic field is applied to it, this sponge-like structure can autonomously rotate itself, producing a stirring motion that mixes chemicals at a molecular level.
The team's nanoreactor incorporates magnetic iron oxide (Fe₃O₄) particles that initiate its rotation, as well as gold (Au) and palladium (Pd) nanocrystals separated in different chambers. This characteristic design facilitates the mixing of catalysts at a nanoscale level, paving the way for the more precise control and enhancement of chemical reactions required to produce specific compounds.
"The nanoreactors are designed by encapsulating magnetic Fe3O4 (~20 nm) in the first chamber, and meantime, Au and Pd nanocrystals are spatially isolated in different domains," explained Ma, Guo and their colleagues.
"Such a nanoreactor generates nanoscale rotation under the rotating magnetic fields and exhibits an order of magnitude activity enhancement in the cascade synthesis of 5,6-dimethylphenanthridinium (96.4% selectivity) compared with conventional macro-stirring. We quantitatively uncovered the rotation-induced enhancement in sequential and reverse transfer of reactive intermediates, consequently revealing the relevance of self-rotation and proximity effects in controlling the catalytic performance."
As part of their study, Ma, Guo and their colleagues used their paddle-like nanoreactor to synthesize the organic compound 5,6-dimethylphenanthridinium, which is widely used to create industrial materials, as well as in the medical and pharmaceutical industries.
The researchers were able to synthesize this compound with a remarkable selectivity of 96.4%, which is significantly higher than the selectivity yielded by conventional macro-stirring techniques.
In the future, the newly introduced nanoreactor could be improved further and applied to the synthesis of other compounds. Moreover, its underlying design could soon inspire the development of similar nanotechnologies that could enhance the efficiency and sustainability of chemical manufacturing or synthesis processes.
More information: Yuzhu Ma et al, Paddle-like self-stirring nanoreactors with multi-chambered mesoporous branches for enhanced dual-dynamic cascade reactions, Nature �������� (2025). .
Journal information: Nature ��������
© 2025 Science X Network