Zeolite nanopore model links crystal size to metal cluster migration and catalyst performance

Stephanie Baum
scientific editor

Robert Egan
associate editor

Extensive industrial catalytic applications have shown that the confined nano-channels of zeolites can precisely regulate molecular diffusion and metal cluster migration, effectively enhancing catalyst activity, selectivity, and stability.
A deep understanding and quantitative description of the coupling between confined "transport and reaction" processes are essential for designing and optimizing industrial zeolite catalysts. However, constructing such a theoretical model poses a significant challenge, largely due to the difficulty in precisely characterizing and describing these confined transport phenomena.
To address this challenge, a research team, led by Prof. Liu Zhongmin and Prof. Ye Mao from the Dalian Institute of Chemical 萌妹社区ics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. Bao Xiaojun and Prof. Zhu Haibo from Fuzhou University, has proposed a theoretical model describing the migration-aggregation behavior of confined metal clusters within individual zeolites.
The study is in Nature.
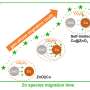
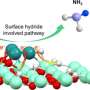
Using advanced first-principles simulations, the researchers investigated the migration and aggregation kinetics of metal clusters within the nanopores of silicate-1 (S-1). This work led to the establishment鈥攆or the first time鈥攐f a theoretical model specifically describing the migration-aggregation process of metal clusters within zeolite nanopores.
This model reveals the dynamic behavior of metal clusters within the S-1 micro-region and quantitatively describes how the crystal size of S-1 affects the spatial and temporal distribution of these clusters. The model's reliability was validated through various in-situ high-spatial-resolution spectroscopic characterizations.
Using this model, researchers discovered that the crystal size of S-1 plays a key role in regulating two competing mechanisms: surface aggregation of metal clusters, which leads to the formation of low-activity metal nanoparticles, and aggregation within the nanopores, which results in high-activity sub-nanometer metal clusters.
Furthermore, the researchers found that when the b-axis length of S-1 exceeds 2 渭m, the extended migration path drives Pt species to aggregate within the pores, forming sub-nanometer Pt clusters. These clusters become effectively locked in place within the nanopores, thereby preventing irreversible catalyst deactivation.
Based on this mechanism, the researchers proposed a strategy to achieve the "migration-aggregation-self-locking" of Pt species by increasing the crystal size of S-1. This approach enabled the creation of an ultra-stable Pt-Sn@MFI catalyst for propane dehydrogenation.
"Our study quantitatively describes metal cluster migration and aggregation within individual zeolites, offering an important theoretical foundation for precisely regulating confined metal cluster migration-aggregation by adjusting zeolite support properties," said Prof. Ye.
More information: Zhikang Xu et al, Pt migration-lockup in zeolite for stable propane dehydrogenation catalyst, Nature (2025).
Journal information: Nature
Provided by Chinese Academy of Sciences