Chemical synthesis: New strategy for skeletal editing on pyridines
A team from the University of M眉nster has introduced a strategy for converting carbon鈥搉itrogen atom pairs in a frequently used ring-shaped compound into carbon鈥揷arbon atom pairs. The method has potential in the quest for active ingredients for new drugs, for example. The results are in Nature Chemistry.
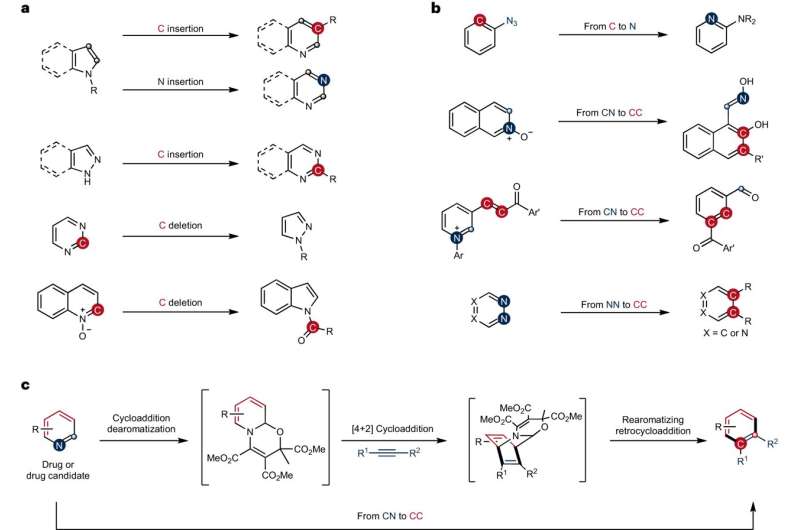
In the field of chemistry, so-called skeletal editing is seen as a method suitable for changing ring-shaped structures precisely by swapping individual atoms. A team of researchers led by Prof. Armido Studer from the Institute of Organic Chemistry at the University of M眉nster has now introduced a new strategy for converting carbon鈥搉itrogen atom pairs in pyridines鈥攁 ring-shaped compound frequently used as a synthesis building block鈥攊nto carbon鈥揷arbon atom pairs. The method has potential in the quest for new drugs and materials that are often based on such molecule rings.
While the ring structure remains intact in the so-called peripheral functionalization of rings鈥攚hich involves for example the attachment of groups of atoms鈥攕keletal editing requires the cleavage of robust bonds between carbon atoms or between a carbon atom and another atom within the ring.
"In organic synthesis," says Studer, "this is considered to be particularly challenging鈥攚e can imagine it as a kind of surgical procedure." Up to now, no synthesis strategy was known which could be used to swap complex pyridines by means of skeletal editing.
In this new approach, the team produced benzenes and naphthalenes with functional groups which are attached precisely to specific positions. Functional groups are groups of atoms that play a decisive role in the properties of a compound.
"The pyridines which we used are inherently inert, making it difficult to modify them," explains post-doc Dr. Qiang Cheng. "We first had to change their specific bonding structure鈥攃arrying out so-called dearomatization鈥攊n order to obtain significantly more reactive intermediates. The subsequent cycloaddition and rearomatization processes ultimately result in the formation of the skeletal-edited compounds."
Debkanta Bhattacharya, a Ph.D. student on Studer's team, adds, "Now, by using a so-called one-pot procedure, we can introduce synthetically valuable and medically significant functional groups to specific positions on rings."
Chemists speak of a one-pot reaction to describe a synthesis in which the reagents needed react with one another in a single vessel. The reaction sequence's mechanism was theoretically analyzed by Dr. Christian M眉ck-Lichtenfeld from the Institute of Organic Chemistry.
More information: Qiang Cheng et al, Skeletal editing of pyridines through atom-pair swap from CN to CC, Nature Chemistry (2024).
Journal information: Nature Chemistry
Provided by University of M眉nster