Ultrafast X-rays capture atomic movements in light-activated catalyst molecules

Lisa Lock
scientific editor

Robert Egan
associate editor

Catalysts facilitate crucial chemical reactions in nature and industry alike. In a subset of them, catalytic activity is triggered by light. For example, when iron pentacarbonyl鈥攁 molecule in which a central iron atom is surrounded by five carbon monoxide groups鈥攊s exposed to light, the iron sheds its carbon monoxide groups one after another, creating spots for other molecules to dock onto during a catalytic reaction.
Although this process has been studied extensively with spectroscopy, a method that shows how energy moves around in molecules, key details of how the catalyst's atoms change structure after being hit by light remain unknown.
Now, writing in the journal Nature Communications, a team led by researchers at the Department of Energy's SLAC National Accelerator Laboratory how they used ultrafast X-rays from the Linac Coherent Light Source (LCLS), combined with recent theoretical advancements, to reveal those atomic motions on a timescale of femtoseconds, millionths of a billionth of a second. The technique could be used to observe speedy atomic motions in more complex catalysts.
"Part of the fun is to make tools that will open new doors," said Adi Natan, principal investigator and staff scientist at the Stanford PULSE Institute, a joint institute of SLAC and Stanford University. "And being able to see how molecular structures evolve with unprecedented detail will allow us to learn something new about the chemistry of molecules."
Getting more out of X-ray scattering data
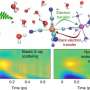
At LCLS, the team shone X-ray pulses on their iron pentacarbonyl sample and analyzed how the X-rays scattered into a detector. Changes in the recorded scattering pattern over time allowed them to determine how the sample's atomic structure responded to the triggering light flashes.
However, the detector's finite size and other experimental constraints limit the amount of information researchers can glean from these scattering signals. Natan said, "Transforming this restricted scattering data into real-space structural information is like trying to see fine details through a funhouse mirror."
To overcome these limitations, researchers typically interpret scattering data by matching simulations of possible molecular structures with the data instead of translating the data directly into real space. This also means the analysis becomes harder the more difficult it is to simulate a molecular structure. The metal center in iron pentacarbonyl, for example, makes simulations of atomic motions very challenging.
To get around that, Natan used a theoretical approach he developed previously that relates the observed scattering patterns to the distances between all possible atomic pairs in the molecule. This allows the structure to be extracted directly from the scattering data without simulations.
Atomic 'spectators' pave the way for more complex analyses
The LCLS study on iron pentacarbonyl was one of the first experimental applications of this new approach, and it allowed the researchers to follow precisely what happened to iron pentacarbonyl as it lost two carbon monoxide groups following a flash of light.
First, the light created vibrations in the molecule that led to the dissociation of one carbon monoxide and a simultaneous rearrangement of the remaining carbon monoxides around the central iron. Then, the second carbon monoxide group was lost with less coordinated movement.
But the research team also observed an effect they hadn't expected. Although the observed vibrations originated in an iron-carbon pair, the wiggling also occurred in many of the other atomic pairs, which acted as "spectators" that amplified the original motion.
Natan said the spectator effect allows tracking of atomic motions throughout the entire molecule by using the motion of the original atomic pair to benchmark the motions of other pairs. And, because the effect does not hinge on the complexity of a molecule, it opens a door to observing much more complex molecules than iron pentacarbonyl.
Combining the structural results with spectroscopy data provides a fuller picture of how chemical reactions unfold鈥攊nsights that could ultimately allow researchers to tailor the performance of catalysts for different applications.
"Understanding how energy flows through molecules and how atoms move in real space and time brings us one step closer to controlling chemical reactions, helping us design materials," Natan said.
In addition to researchers from the Stanford PULSE Institute, LCLS and Stanford, the team included members from DOE's Pacific Northwest National Laboratory; Brown University; Western Connecticut State University; Stockholm University, Sweden; and TCG Centers for Research and Education in Science and Technology, India.
More information: Aviad Schori et al, Real-space observation of the dissociation of a transition metal complex and its concurrent energy redistribution, Nature Communications (2025).
Journal information: Nature Communications
Provided by SLAC National Accelerator Laboratory