Cobalt catalyst rivals platinum in key industrial reaction

Stephanie Baum
scientific editor

Robert Egan
associate editor

Propane dehydrogenation is a key industrial route to producing propylene without relying on oil. However, its current production processes rely heavily on precious-metal catalysts such as Pt-based materials. Developing efficient alternatives using Earth-abundant metals has remained a challenge.
In a study in Nature Catalysis, Prof. Xiao Jianping's group from the Dalian Institute of Chemical ��������ics (DICP) of the Chinese Academy of Sciences, and collaborators, have developed a high-performance cobaltosilicate zeolite catalyst (CoS-1) via a hydrothermal synthesis method.
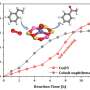
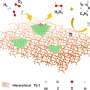
The catalyst has solely tetrahedral cobalt sites and none of the unstable cobalt species, achieving a propylene productivity as high as 9.7 kgC3= kgcat–1 h–1, surpassing that of an industrial PtSn/Al2O3 catalyst.
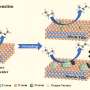
The synthesis method involved a gel composed of cobalt salts, tetraethyl orthosilicate, tetrapropylammonium hydroxide (TPAOH), urea, and water, followed by crystallization at 180°C. After calcination to remove the organic template and triple nitric acid washing at 80°C to eliminate excess cobalt species, the resulting CoS-1 catalyst contained only tetrahedral cobalt sites without unstable Co species.
Through density functional theory calculations and ab initio molecular dynamics simulations, researchers studied the stability of different active centers, and uncovered the mechanism behind the high performance of the CoS-1 catalyst.
They revealed that the flexible zeolite framework obviously lowered the dehydrogenation barriers at isolated cobalt sites due to entropic effects, resulting in a lower barrier of propane dehydrogenation than a Pt3Sn alloy.
Microkinetic simulations further showed that while CoS-1 had a lower dehydrogenation barrier, its overall reaction rate at initial conversions was slightly lower than that of Pt3Sn, due to reduced propane concentration at isolated Co sites—an effect of entropy loss during diffusion into the zeolite channels.
The CoS-1 developed in this study exhibits excellent long-time stability. Researchers have proven that this can be attributed to the non-bonding adsorption of propylene within the zeolite, which enables rapid product desorption and reduces coke formation.
More information: Hang Zhou et al, Cobaltosilicate zeolite beyond platinum catalysts for propane dehydrogenation, Nature Catalysis (2025).
Journal information: Nature Catalysis
Provided by Chinese Academy of Sciences