Theoretical understanding of electrocatalysis beyond thermodynamic analysis
As green and sustainable development relies on renewable energy, electrocatalysis has become a key technology. Advanced theoretical study is an important means to fundamentally understanding electrocatalytic reactions. Highly efficient methods of carbon-neutralization (eCO2RR), reverse artificial nitrogen cycle (RANC), and oxygen chemistry (OER and ORR) can all be driven by electrocatalysis.
In this vein, the computational hydrogen electrode (CHE) model developed by N酶rskov et al can describe the influence of electrode potential on the reaction free energies by shifting the chemical potential of proton-electron couple. It has been widely used for estimating the thermodynamics in electrocatalysis.
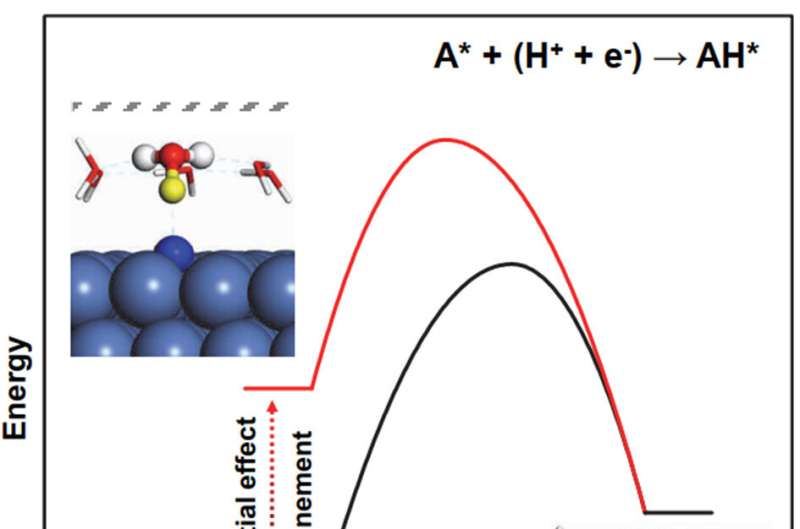
The study of interfacial kinetics is vital for the understanding of catalytic reaction and design of catalysts. Calculating the potential-dependent barriers of a proton-electron coupled transfer reaction is an important step of microkinetic modeling in electrocatalysis.
The charge-extrapolation method based on the capacitor model can easily and accurately obtain the barrier under a specific potential, so as to fully consider the influence of the electrode potential on the reaction. The interface microenvironment can also affect surface reactions, and considering the competition among multiple paths can provide a more inclusive understanding.
In a perspective published in Chinese Journal of Catalysis, a research team led by Prof. Jianping Xiao from Dalian Institute of Chemical 萌妹社区ics, CAS summarized the efforts towards calculating potential-dependent barriers on the basis of charge-extrapolation method.
Electrochemical barriers and potential effects are essential for a more accurate description of reaction mechanism and activity. Meanwhile, consideration of competitive reaction paths is also one of the important aspects, as novel insights and anomalous volcano trend can be obtained. Finally, confined microenvironment shows a possibility of tuning the capacitance at water-electrode interface, resulting in a possibility to improve reaction activity, which could open a new avenue for electrode design in electrocatalysis.
More information: Huan Li et al, Theoretical understanding of electrocatalysis beyond thermodynamic analysis, Chinese Journal of Catalysis (2022).
Provided by Chinese Academy of Sciences





















