Addressing global warming with new nanoparticles and sunshine
Harvesting sunlight, researchers of the Center for Integrated Nanostructure 萌妹社区ics, within the Institute for Basic Science (IBS, South Korea) published in Materials Today ("Phase-Selective Highly Efficient Nanostructured Metal-decorated Hybrid Semiconductors for Solar Conversion of CO2 to Absolute CO Selectivity") a new strategy to transform carbon dioxide (CO2) into oxygen (O2) and pure carbon monoxide (CO) without side-products in water. This artificial photosynthesis method could bring new solutions to environmental pollution and global warming.
While, in green plants, photosynthesis fixes CO2 into sugars, the artificial photosynthesis reported in this study can convert CO2 into oxygen and pure CO as output. The latter can then be employed for a broad range of applications in electronics, semiconductor, pharmaceutical, and chemical industries.
The key is to find the right high-performance photocatalyst to help the photosynthesis take place by absorbing light, convert CO2, and ensuring an efficient flow of electrons, which is essential for the entire system.
Titanium oxide (TiO2) is a well-known photocatalyst. It has already attracted significant attention in the fields of solar energy conversion and environmental protection due to its high reactivity, low toxicity, chemical stability, and low cost.
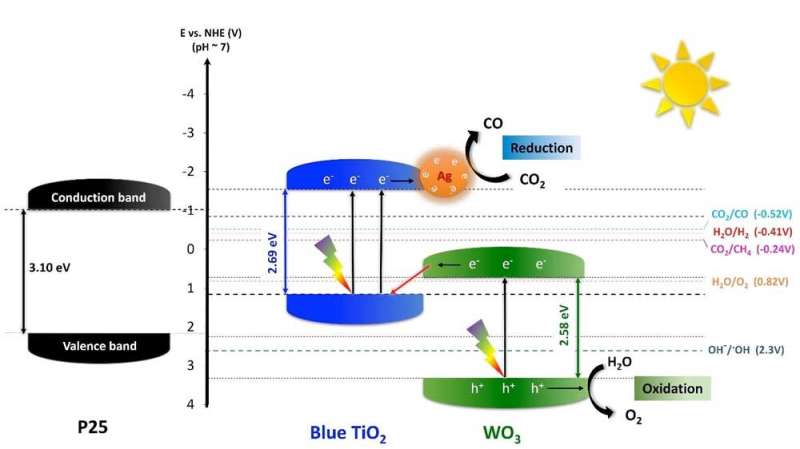
While conventional TiO2 can absorb only UV light, the IBS research team reported previously two different types of blue-colored TiO2 (or "blue titania") nanoparticles that could absorb visible light thanks to a reduced bandgap of about 2.7 eV.
They were made of ordered anatase/disordered rutile (Ao/Rd) TiO2 (called, HYL's blue TiO2-I) ("An order/disorder/water junction system for highly efficient co-catalyst-free photocatalytic hydrogen generation"), and disordered anatase/ordered rutile (Ao/Rd) TiO2 (called, HYL's blue TiO2-II) ("Visible-Light-Driven, Metal-Free CO2 Reduction"), where anatase and rutile refer to two crystalline forms of TiO2 and the introduction of irregularities (disorder) in the crystal enhances the absorption of visible and infrared light.
For the efficient artificial photosynthesis for the conversion of CO2 into oxygen and pure CO, IBS researchers aimed to improve the performance of these nanoparticles by combining blue (Ao/Rd) TiO2 with other semiconductors and metals that can enhance water oxidation to oxygen, in parallel to CO2 reduction into CO only.
The research team obtained the best results with hybrid nanoparticles made of blue titania, tungsten trioxide (WO3), and 1percent silver (TiO2/WO3鈥揂驳).
WO3 was chosen because of the low valence band position with its narrow bandgap of 2.6 eV, high stability, and low cost. Silver was added because it enhances visible light absorption, by creating a collective oscillation of free electrons excited by light, and also gives high CO selectivity.
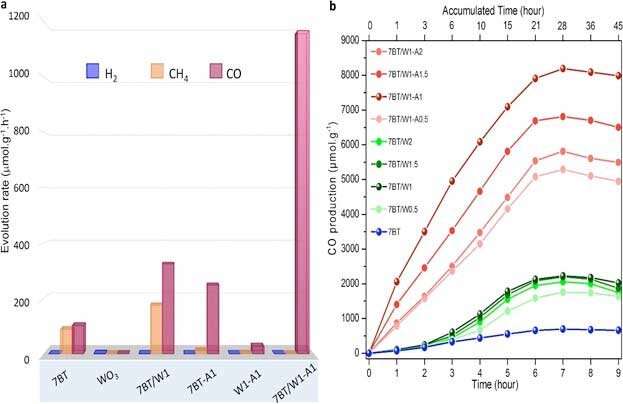
The hybrid nanoparticles showed about 200 times higher performance than nanoparticles made of TiO2 alone and TiO2/WO3 without silver.
Starting from water and CO2, this novel hybrid catalyst produced O2 and pure CO, without any side products, such as hydrogen gas (H2) and metane (CH4). The apparent quantum yield that is the ratio of several reacted electrons to the number of incident photons was 34.8 percent, and the rate of reacted electrons 2333.44 碌mol g-1h-1. The same measurement was lower for nanoparticles without silver (2053.2 碌mol g-1h-1), and for nanoparticles with only blue TiO2 (912.4 碌mol g-1h-1).
More information: Chau T.K. Nguyen et al. Highly efficient nanostructured metal-decorated hybrid semiconductors for solar conversion of CO2 with almost complete CO selectivity, Materials Today (2020).
Kan Zhang et al. An order/disorder/water junction system for highly efficient co-catalyst-free photocatalytic hydrogen generation, Energy & Environmental Science (2015).
Hee Min Hwang et al. Phase-Selective Disordered Anatase/Ordered Rutile Interface System for Visible-Light-Driven, Metal-Free CO2 Reduction, ACS Applied Materials & Interfaces (2019).
Journal information: Materials Today , Energy & Environmental Science , ACS Applied Materials and Interfaces
Provided by Institute for Basic Science