Explaining how 2-D materials break at the atomic level

We are familiar with cracks in big or small three-dimensional (3-D) objects, but how do thin, two-dimensional (2-D) materials crack? 2-D materials like molybdenum disulfide (MoS2), have emerged as an important asset for future electronic and photoelectric devices. However, the mechanical properties of 2-D materials are expected to differ greatly from 3-D materials. Scientists at the Institute for Basic Science (IBS) have published the first observation of 2-D MoS2 cracking at the atomic level in Nature Communications. This study is expected to contribute to the applications of new 2-D materials.
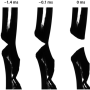
When a certain force is applied to a material, a crack forms. Less obvious is how to explain and predict the shape and seriousness of a crack from a physics point of view. Scientists want to investigate which fractures are likely to expand and which are not. Materials are described as ductile or brittle: Ductile materials, like gold, withstand large strains before rupturing; brittle materials, like glass, can absorb relatively little energy without elongation and deformation before breaking suddenly. At the nano-level, atoms move more freely in ductile materials than in brittle materials; so in the presence of a pulling force (tensile stress) they can go out of position from the ordered crystal structure; in technical terms, they dislocate. So far, this explanation (the Griffith model) has been applied to cracking phenomena in bulk, but it lacks experimental data at the atomic or nano-scale.
In this study, IBS scientists observed how cracks propagate in 2-D MoS2 after a pore was formed either spontaneously or with an electron beam. "The most difficult point {of the experiments} was to use the electron beam to create the pore without generating other defects or breaking the sample," explains Thuc Hue Ly, first author of this study. "So we had to be fast and use a minimum amount of energy."
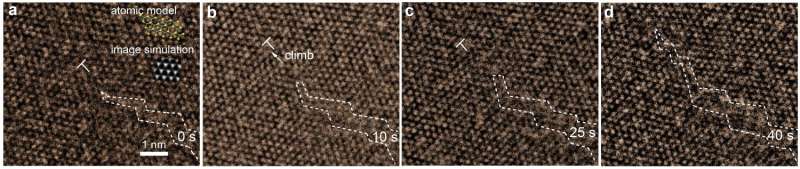
The atomic observations were done using real-time transmission electron microscopy. Surprisingly, even though MoS2 is a brittle material, the team saw atom dislocations three to five nanometers (nm) away from the front line of the crack, or crack tip. This observation cannot be explained with the Griffith model.
In order to create conditions that represent the natural environment, the sample was exposed to ultraviolet (UV) light. This caused the MoS2 to oxidize; atom dislocations occurred more rapidly and the stretched region expanded to five to 10 nm from the crack tip.
"The study shows that cracking in 2-D materials is fundamentally different from cracking in 3-D ductile and brittle materials. These results cannot be explained with the conventional material failure theory, and we suggest that a new theory is needed," explained Professor LEE Young Hee (CINAP).
More information: Nature Communications,
Journal information: Nature Communications
Provided by Institute for Basic Science