July 27, 2016 report
Linking superatoms to make molecules to use as building blocks for new materials
(萌妹社区)鈥擜 team of chemists at Columbia University in New York has for the first time linked together superatoms to make new types of molecules. As they note in their paper published in the journal Nano Letters, the technique may be used in the future to create unique materials with applications in magnetics and electronics.
Superatoms are actually clusters of atoms that appear to exhibit many of the same properties as elemental atoms (their electrons form a shell around a middle core), but because they are made of many particles they can have their properties changed by changing their parts鈥攚hich makes them ripe for experimentation. In this new effort, the researchers have built very simple molecules using just two or three superatoms suggesting that it might be possible to create exotic materials from them.
Superatoms are generally created by heating material that causes vapor to form鈥攁s the vapor cools, the atoms condense naturally to form superatoms in magic number combinations. Prior efforts to create molecules from them have generally involved taking advantage of self-assembly processes and because of that have not been systematic.
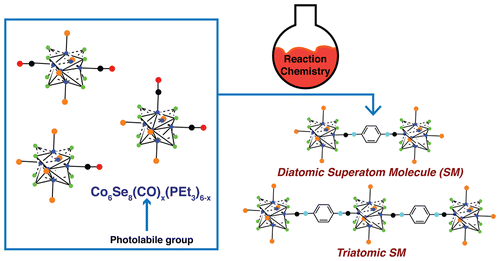
In this new effort the team configured their super-atoms with a core of eight selenium atoms and six cobalt atoms. Then they added ligands from several other atoms to serve as a means to bond the atoms together (because their surface was well defined) resulting in triatomic or diatomic molecules. The idea, the researchers suggest, is to learn more about the ways such molecules can be made in order to create something that is superior to the individual pieces. They report that the super-atoms can be made to bond in ways very similar to traditional atoms and that they were able to replace the carbonyl groups used initially with phosphine moieties or isocyanide, which made it possible to regulate the length and type of chemical bridges that were forged. They expect that any future materials made from the new kinds of molecules will be conductive, magnetic or both鈥攁nd that they will be customizable.
The group has plans to build larger molecules while tinkering with their properties to see what sorts of materials might be made from them.
More information: Anouck M. Champsaur et al. Building Diatomic and Triatomic Superatom Molecules, Nano Letters (2016).
Abstract
In this study, we have developed a method to create Co6Se8 superatoms in which we program the metal鈥搇igand bonds. We exclusively form the Co6Se8 core under simple reaction conditions with a facile separation of products that contain differential substitution of the core. The combination of Co2(CO)8 and PR3 with excess Se gives the differentially and directionally substituted superatoms, Co6Se8(CO)x(PR3)(6鈥搙). The CO groups on the superatom can be exchanged quantitatively with phosphines and isonitriles. Substitution of the CO allows us to manipulate the type and length of chemical bridge between two redox-active superatomic centers in order to modulate intersuperatomic coupling. Linking two superatoms together allows us to form the simplest superatom molecule: a diatomic molecule. We extend the superatom molecule concept to link three superatoms together in a linear arrangement to form acyclic triatomic molecules. These superatom molecules have a rich electrochemical profile and chart a clear path to a whole family of superatom molecules with new and unusual collective properties.
Journal information: Nano Letters
漏 2016 萌妹社区