This article has been reviewed according to Science X's and . have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Optimized enzymes promote nitrogen-nitrogen bonds to generate bioactive compounds

Microorganisms generate all sorts of materials that could serve as potential agents for combating bacteria and fungi. An international research team has identified and optimized enzymes that can specifically generate a certain functional group of these natural substances, in turn expanding the toolkit of potential agents.
The researchers, led by Professor Sandy Schmidt (University of Groningen, Netherlands) and Professor Dirk Tischler (Ruhr University Bochum, Germany), report in the journal ACS Catalysis.
Bacteria and other microorganisms generate a range of substances that are not necessary for their survival. However, these "secondary metabolites" do provide them with environmental benefits. For example, they aid in communication or defense against other organisms.
"The breadth of these compounds is far from being fully understood," says Tischler. The researchers hope to be able to use these natural substances as bioactive compounds in medications, or as a blueprint for synthesizing new agents.
"Penicillin is one such secondary metabolite," Tischler clarifies.
Effective against fungi and certain bacteria
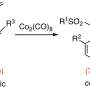
Of particular interest are the kutznerides, which are effective against some fungi as well as Gram-positive bacteria. Their effectiveness is determined by functional groups. The research team is currently focusing on kutznerides, whose functional group contains a bond between two nitrogen atoms. Experts refer to this functional group as hydrazine.
"These compounds do occur in nature," says Tischler. "But we have been able to influence them and their environment in a targeted manner."
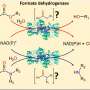
The researchers have identified previously unknown enzymes that initiate the nitrogen-nitrogen bond and connect the atoms. They further optimized the enzymes via mutagenesis so that they could convert different substrates. After this, the various enzymes were consolidated into a cascade.
"We were able to show that non-natural substrates could be converted into 5- and 6-ring cyclical ring structures including a nitrogen-nitrogen bond," reports Tischler.
In some instances, a chiral center that is crucial for the later syntheses of pharmaceutical substances was inserted.
More information: Yongxin Li et al, Access to Nitrogen鈥搉itrogen Bond-Containing Heterocycles Through Substrate Promiscuity of Piperazate Synthases, ACS Catalysis (2025).
Journal information: ACS Catalysis
Provided by Ruhr-Universitaet-Bochum