This article has been reviewed according to Science X's and . have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Lethal bacteria use sugar-sensing mechanism to recognize and infect cells

Scientists led by Karla Satchell, Ph.D., the Anne Stewart Youmans Professor of Microbiology at Northwestern University, have discovered previously unknown molecular mechanisms that help a type of food-borne bacteria recognize host cells and initiate infection on the cell surface, according to a recent study in Science Advances.
Multifunctional autoprocessing repeats-in-toxin (MARTX) toxins are secreted by bacteria and support the spread of many Gram-negative bacteria, including Vibrio vulnificus, a lethal food-borne bacterium commonly found in raw or undercooked shellfish.
MARTX toxins use precise intracellular mechanisms to infiltrate host cells and cause life-threatening infections, as detailed in previous work led by Satchell. However, the mechanisms these toxins use to identify and recognize host cells have remained poorly understood.
"We wanted to understand the surface interactions and why this toxin is so pervasive," said Satchell, who is also a professor of Microbiology-Immunology and director of the Center for Structural Biology of Infectious Diseases.
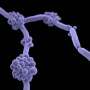
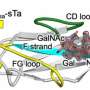
In the current study, the investigators used a combination of cellular techniques to map the small portion of the large MARTX toxin from Vibrio vulnificus that directly interacts with the surface of cells.
The scientists found this domain binds N-acetylglucosamine (GlcNAc) (an amino sugar and key building block of complex glycans on the exposed surfaces of epithelial cells) to N-glycans (sugar molecules that decorate proteins on the surface of the host cell) with select preference for the L1CAM protein and clusters of N-glycans on host cell surfaces.
"Different cell types have different kinds of sugars on them, and this is one way that toxins can discriminate one cell versus a different kind of cell," Satchell said.
The scientists also found that this domain is essential for Vibrio vulnificus infection during intestinal infection.
"We showed for the first time that this ubiquitous N-glycan structural motif present on diverse host surfaces can be recognized by a virulence factor, which explains why MARTX toxin is so pervasive," said Jiexi Chen, a graduate student in the Driskill Graduate Program in Life Sciences (DGP) and lead author of the study.
Future work, according to Chen, will include understanding the structure of how this protein binds to the glycan, as well as identifying other receptor binding domains that promote infection.
More information: Jiexi Chen et al, Vibrio MARTX toxin binding of biantennary N-glycans at host cell surfaces, Science Advances (2025).
Journal information: Science Advances
Provided by Northwestern University