Key difference in how cells interact could aid in development of more targeted drugs

Stephanie Baum
scientific editor

Robert Egan
associate editor

Talin is a protein that plays an important role in the immune system by activating integrins, receptors that help cells attach to one another. Now a new study by Fox Chase Cancer Center researchers shows how talin has distinct binding modes for two types of integrins that are important in blood cells. It also highlights how switching modes can enhance the integrins, potentially making them stronger.
The work is in the journal Cell Reports.
The findings are important because they could be used to make therapies more targeted, such as by activating one type of integrin without affecting the other. These approaches could be applied to treating autoimmune diseases, targeting tumor metastasis, or enhancing immunotherapies to fight cancer.
"Often, when treating blood cells, you don't want to eliminate their basic physiological function, you just want to fine-tune or modulate them more precisely," said senior author Jinhua Wu, Ph.D., a Professor in the Cancer Signaling and Microenvironment Research Program at Fox Chase. "By better understanding the talin molecule's conformation, we can target the sites that do not directly interact with integrin, which can better stabilize its function."
Integrins are receptors that sit on the surface of the cell. In addition to binding with other cells, they can help a cell attach to extracellular surfaces in the surrounding environment. There are many subtypes of integrins, and talin connects with each subtype a bit differently.
For the new paper, Wu's team compared two common integrin subtypes. Beta-2 integrins are important for lymphocytes, white blood cells that help with immune response. Beta-3 integrins help support platelets, which are important for blood clotting and wound healing.
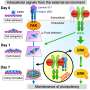
By studying the detailed molecular structure of the interface between talin and integrin, the team identified two distinctly different binding modes for beta-2 and beta-3. They also found that mutations at this interface could shift the talin from one binding mode to the other. Finally, they showed that shifting from the beta-2 to the beta-3 binding mode increased talin activity, enhancing its ability to bind.
In addition, researchers proposed a "seesaw" model, suggesting that talin activity could be modulated by "wobbling" its subdomain from one end to the other.
Wu said the research represents an important step forward in better understanding how talin and integrins interact, and how that interaction can be harnessed for therapeutic benefit.
"At the basic science level, we know that we cannot treat all integrins the same way, because there are different types," he said. "This gives us more insight into how talin specifically recognizes different integrin types and how we can potentially modulate that process."
The researchers are planning similar studies to understand how talin interacts with more integrin subtypes, some of which serve important functions in other types of cells beyond blood cells. They also hope to take a closer look at beta-2 and beta-3 binding to identify small molecule drugs that could be used to stabilize the binding mode, potentially making therapies more effective.
More information: Tong Gao et al, Molecular basis of 尾2 integrin activation by talin unveils subunit-specific mechanisms of integrin signaling, Cell Reports (2025).
Journal information: Cell Reports
Provided by Temple University