This article has been reviewed according to Science X's and . have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
How a flexible protein domain links gene transcription and RNA processing

In a new study in Cell Reports, researchers at the University of Freiburg reveal how a disordered protein segment helps connect two key steps of gene expression: the reading of genes and the editing of their RNA products.
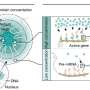
The protein in question, TAF2, is part of the general transcription factor TFIID. A specific, so-called intrinsically disordered region (IDR) within TAF2 turns out to function as a built-in positioning signal that guides the protein to distinct regions inside the cell nucleus.
The study highlights that flexible protein regions like the IDR of TAF2 not only shape the spatial organization of molecular processes, but could also act as key regulators of specific functions through targeted localization to nuclear speckles—a mechanism that may also be relevant in disease-related processes.
Led by Dr. Tanja Bhuiyan, corresponding author and postdoctoral researcher at the Institute of Experimental and Clinical Pharmacology and Toxicology, University of Freiburg, and the Cluster of Excellence CIBSS–Centre for Integrative Biological Signalling Studies, the study uncovers a new regulatory role for a type of protein domains common in many nuclear proteins, but until now only poorly understood.
A flexible signal that guides molecular coordination
The IDR in TAF2 steers the protein away from active gene promoters, where it participates in transcription initiation, to nuclear speckles—liquid-like compartments that concentrate factors for RNA processing. Using a combination of advanced imaging, genome-wide sequencing analyses, and proteomics, the team shows that TAF2 can move dynamically between different nuclear sites, adopting different roles depending on its location.
"We found that TAF2 doesn't just operate at gene promoters as part of the classical TFIID complex," explains Tanja Bhuiyan. "Instead, this flexible region allows it to move between different nuclear compartments—enabling it to interact with RNA-processing machinery and help shape how gene messages are finalized."
The researchers showed that nuclear TAF2 exists in three functional pools: within canonical TFIID at promoters; in non-canonical complexes with the splicing factor SRRM2; and concentrated in nuclear speckles. When the IDR was removed, TAF2 failed to localize to nuclear speckles and accumulated more strongly at gene promoters. Surprisingly, this shift did not significantly change global gene activity, but it altered how certain RNA transcripts were processed—a mechanism known as alternative splicing, which enables cells to produce different proteins from a single gene.
From structure to impact: Linking transcription to RNA editing
"This suggests that the spatial routing of TAF2 doesn't switch genes on or off in a binary way," explains Prof. Dr. Sebastian Arnold, last author of the study and group leader at the Institute of Experimental and Clinical Pharmacology and Toxicology, University of Freiburg, and CIBSS—Centre for Integrative Biological Signalling Studies. "It rather modulates how information is processed at the RNA level—a more subtle but potentially powerful form of gene regulation."
These insights contribute to a growing understanding of how cells coordinate complex molecular processes in space and time. Rather than functioning exclusively through linear signaling cascades, many regulatory pathways rely on dynamic spatial compartmentalization and molecular flexibility.
The study also highlights the emerging importance of intrinsically disordered protein regions (IDRs). Once considered structurally ambiguous, these flexible sequences are now recognized as key elements in the formation of biomolecular condensates and the regulation of functional specificity. The IDR of TAF2 includes a conserved stretch of histidines and lysines, supporting the idea that speckle targeting through phase separation may represent a broader principle in nuclear gene regulation.
While the current study did not directly address disease mechanisms, several of the alternative splicing events influenced by TAF2 affected genes involved in neurodevelopment and membrane transport. These findings suggest that TAF2's spatial dynamics could have broader biological relevance. Future research may explore whether this regulatory mechanism plays a role in cell identity, stress responses, or pathological conditions.
More information: Tanja Bhuiyan et al, TAF2 condensation in nuclear speckles links basal transcription factor TFIID to RNA splicing factors, Cell Reports (2025).
Journal information: Cell Reports
Provided by University of Freiburg