This article has been reviewed according to Science X's and . have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Controlling contaminants inside nanopores holds promise for desalination, carbon dioxide storage and porous catalysts

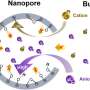
Natural and engineered systems have a whole world of chemistry inside their tiny pores鈥攌nown as nanopores鈥攖hat changes depending on the chemical functional groups inside.
A team of chemical engineers and materials scientists in the McKelvey School of Engineering at Washington University in St. Louis has found a way to precisely control contaminants in nanopores that would ultimately benefit desalination techniques used in water treatment; carbon dioxide storage; and porous catalysts used in other manufacturing processes.
The research is in the journal ACS Applied Materials & Interfaces.
Young-Shin Jun, a professor of energy, environmental and chemical engineering, and Srikanth Singamaneni, the Lilyan & E. Lisle Hughes Professor in the Department of Mechanical Engineering and Materials Science, and their teams have published results of research that sheds light on how chemical functional groups influence the concentration of ions and pH inside nanopores.
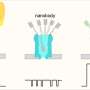
Singamaneni's lab created a plasmonic nanosensor to measure concentrations of protons and ion contaminants in nanopores. The nanosensor provided information about ion-nanopore interactions that tells the researchers how certain contaminants are precisely and selectively controlled.
"Learning the local concentrations of precursor ions, such as protons, anions and cations, in differently functionalized nanopores can improve our understanding of crucial catalytic reactions in those nanopores, allowing more precise control in pore engineering," Jun said.
It is a continuation of their work published in 2022 in Chem that found that in higher-salinity solutions, the pH inside nanopores can be as much as 100 times more acidic than in the bulk solution.
The team used in situ surface-enhanced Raman spectroscopy (SERS) combined with a specially designed core-shell plasmonic nanosensor composed of a gold nanorod and functionalized mesoporous silica to measure pH and ion concentrations within nanopores close to the gold nanorod surface. They examined inside functionalized nanopores for several anions, including phosphate, nitrate, sulfate and arsenate, and cations, including mercury, lead and copper鈥攁ll critical water constituents or contaminants.
They compared their concentrations in the nanopores with those in bulk concentrations. In pristine or hydrophobic nanopores, they unexpectedly found enhanced anion concentration and suppressed cation concentration compared with bulk concentrations. However, in hydrophilic nanopores, such as those in amine, thiol and carboxyl, they found that the pH depended on the acidities of chemical functional groups, and the heavy metal concentrations strongly affected by the strength of chemical interactions with the functional groups.
"Integrating functionalized porous materials with plasmonic nanosensors is a universal and powerful approach to understanding the unusual physical, chemical and biological properties of nanoporous materials," Singamaneni said. "This work establishes a methodology that can be easily extended to other porous materials."
"This finding will help us determine how to make materials that can be used on a broader scale," Jun said. "By understanding nanopore chemistry, we can design better materials and comprehend the reactions that occur within the tiny yet critical world of nanopores.
"Interestingly, many exciting reactions take place in areas that are not directly visible and remain hidden. The pH, chemical species and their concentrations in these reactions often differ from those in the bulk solution. This new insight helps us to understand unexpected reactions within nanoporous systems."
More information: Yaguang Zhu et al, Chemical Functional Groups Regulate Ion Concentrations and pHs in Nanopores, ACS Applied Materials & Interfaces (2025).
Journal information: ACS Applied Materials and Interfaces , Chem
Provided by Washington University in St. Louis