This article has been reviewed according to Science X's and . have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Advanced gene editor enables more precise insertion of complete genes

Ask scientists which gene-editing tool is most needed to advance gene therapy, and they'd probably describe a system that's now close to realization in the labs of Samuel Sternberg at Columbia University Vagelos College of ��������icians and Surgeons and David Liu at the Broad Institute of MIT and Harvard.
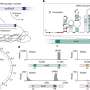
The gene editor—called evoCAST—goes a long way toward solving a problem that has confounded the development of gene therapies from the field's beginnings: How to add long stretches of DNA to defined locations in the human genome without creating unwanted modifications.
The latest iteration of the editor, which utilizes complex enzymes found in bacteria, can be programmed to insert an entire gene—or multiple genes—into a specific location in the human genome with an efficiency suitable for gene therapy. Details of the editor are described in a published in Science.
The need for an advanced gene editor
CRISPR-Cas, viruses, and other editing systems have enabled dozens of genetic medicines now being developed for patients, but all current methods have drawbacks. Some methods are precise but only make small corrections. Viruses, the most frequently used method in gene therapy, can insert complete genes, but do so randomly while activating immune responses.
A tool like evoCAST could make gene therapy more reliable and efficient, particularly for diseases like cystic fibrosis and hemophilia that are caused by any one of thousands of different mutations.
"Hundreds to thousands of different mutations in the CFTR gene can cause cystic fibrosis, for example, so an inordinate number of distinct gene-editing drugs would be needed to ensure each patient could be treated," says Sternberg. "Instead, something like evoCAST could enable a single gene therapy that inserts a complete and healthy gene into the patient's genome.
"There's more work to be done, but evoCAST represents a milestone in the development of these systems for permanently installing a complete, healthy gene, regardless of the underlying genetic defect."
The new system could also enable simpler and more accurate gene editing in other medical and research applications, including the production of CAR T-cell therapies for cancer treatment, and transgenic cell lines and model organisms needed for biomedical research.
New editor developed from 'jumping genes'
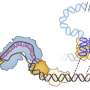
evoCAST is based on a natural system the Sternberg lab discovered several years ago in bacteria that allows genes to jump into new locations in the bacterial genome. (Jumping genes—also known as transposons—can benefit a species by generating genetic diversity).
The lab recognized that several features of CASTs (CRISPR-associated transposases) made them attractive as potential gene editing systems. One advantage is the ability to insert large pieces of DNA without breaking the chromosome in the process, which can introduce serious, unintended errors. Another is the system's "programmability," which directs insertions to any location in the genome specified by the researcher.
Adapting the bacterial system for use in human cells proved challenging. Sternberg's graduate student, George Lampe, successfully developed the system to work in human cells, but early versions of the technology functioned with low efficiency.
Sternberg had expected the difficulty. "CAST systems exist to help mobile genes jump around the genome over evolutionary timescales. They're not under selective pressure to act efficiently, so we reasoned there would be a greater need to squeeze more activity out of these systems compared to CRISPR-Cas9, which evolved into a very potent and efficient system to save bacteria from viral infections."
Artificial evolution improves gene editing
Instead of guessing what changes could improve their system, Sternberg and Lampe turned to David Liu, a molecular biologist and organic chemist at Harvard and the Broad Institute, who established a laboratory technique, PACE, that accelerates the evolution of proteins. Lampe pushed the performance of the system to a point that made PACE a viable option, and Isaac Witte and Simon Eitzinger, two graduate students in Liu's lab, moved the system into PACE, which enabled hundreds of rounds of evolution to be performed with minimal intervention.
"PACE turbocharges evolution and improves enzymes beyond what researchers can typically accomplish with targeted, rationally designed modifications," Lampe says. "The mutations acquired through PACE vastly improved the performance of the entire CAST system."
After hundreds of evolutionary generations, the new evoCAST system is able to edit 30% to 40% of cells, a huge increase from the original system's lower editing rates.
Next steps
The evoCAST system has already achieved efficiencies that are suitable for some gene editing and gene therapy applications, and the researchers are looking to begin testing their system in more relevant model systems.
At the same time, the team is continuing to make improvements, including changes to other evoCAST components, to further improve editing efficiencies.
But one of the biggest challenges right now for evoCAST, and other large DNA editing tools under development, is delivery.
"How do we actually get these tools and their payloads into the cells or tissues of interest?" Sternberg says. "That's a challenge that many of us in the field are facing."
More information: Isaac P. Witte et al, Programmable gene insertion in human cells with a laboratory-evolved CRISPR-associated transposase, Science (2025). .
Journal information: Science
Provided by Columbia University Irving Medical Center