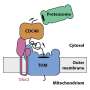
The overall structure of the Orf2971-FtsHi motor complex. A-B, Side views of Orf2971-FtsHi motor complex along the inner envelope membrane (IEM, represented by the orange dotted lines). The protein subunits are painted in different colors. IMS, intermembrane space. C, Top view of Orf2971-FtsHi motor complex from the side of the intermembrane space. D, Bottom view of the Orf2971 motor complex from the stromal side. Credit: Li Mei's group
The TOC (Translocon at the Outer envelope of Chloroplasts)-TIC (Translocon at the Inner envelope of Chloroplasts) supercomplex is responsible for mediating the transport of most precursor proteins from the cytoplasm into the chloroplast. The chloroplast protein transport system includes a motor protein complex, which plays a critical driving role in the translocation of precursor proteins across the chloroplast membrane.
However, little is known about the protein composition and the architecture of the motor complex, as well as the details of the precursor protein translocation.
A research team from the Chinese Academy of Sciences elucidated the high-resolution structure of the Orf2971-FtsHi complex, a chloroplast motor complex from Chlamydomonas reinhardtii. The study reveals the highly complex protein composition and assembly details of the complex, and explores the potential translocation pathway of precursor proteins.
The study, conducted by Prof. Li Mei's team from the Institute of Biophysics of the Chinese Academy of Sciences, and Prof. Yang Wenqiang's team from the Institute of Botany is in Molecular Plant.
The Orf2971-FtsHi complex is a structure with 20 subunits formed by 19 proteins, spanning the chloroplast inner membrane and extending into the intermembrane space as well as the stromal side.
Orf2971, encoded by the chloroplast genome, has a total molecular weight of approximately 341 kDa and is originated from the FtsH protease. Acting as a scaffold, Orf2971 directly interacts with 17 of the other 19 protein subunits, which is crucial for the assembly and stability of the entire complex.
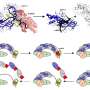
The possible interaction mode between TOC-TIC and Orf2971-FtsHi motor. Credit: LI Mei's group
The structure reveals that five FtsH-like proteins (Orf2971, Ctap1, Fhl1, Fhl2, and Fhl3) form a heterohexameric double-layered disk-like structure (comprising Orf2971, Ctap1, Fhl1, Fhl2, and two identical Fhl3 proteins).
This structure provides the pulling force required for precursor protein translocation by hydrolyzing ATP. The remaining 14 protein subunits assist in complex assembly and stability, and participate in precursor protein transport and regulation.
Using surface plasmon resonance (SPR) and Gaussian accelerated molecular dynamic simulation, the researchers demonstrated a direct interaction between the Tic22 protein in the motor complex and the Toc75 protein in the TOC-TIC complex.
Based on this study, the researchers proposed two models for the association of these complexes, offering significant insights into the coordination between the TOC-TIC supercomplex and the motor protein complex in precursor protein translocation processes and mechanisms. This study provides an important structural basis for understanding the process of the transport of precursor proteins.
More information: Ning Wang et al, Architecture of the ATP-driven motor for protein import into chloroplasts, Molecular Plant (2024).
Journal information: Molecular Plant
Provided by Chinese Academy of Sciences