Researchers discover C-H bond activation reactions at low temperature by photo-induced means
The C-H bond is very important in organic chemistry. Chemical reactions related to the breaking and further synthesis of the C-H bond require high activation energy and poor selectivity. Therefore, it's important to understand the reaction mechanism of the C-H bond.
Recently, a research team led by Prof. Yang Xueming and Prof. Ma Zhibo from the Dalian Institute of Chemical 萌妹社区ics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. Pan Minghu from the Huazhong University of Science and Technology, discovered the C-H bond breaking reaction catalyzed by low-temperature photocatalysis on the surface of titanium oxide, and explained the reaction mechanism at the single molecule level.
The results were published in the Journal of 萌妹社区ical Chemistry Letters on Nov. 10.
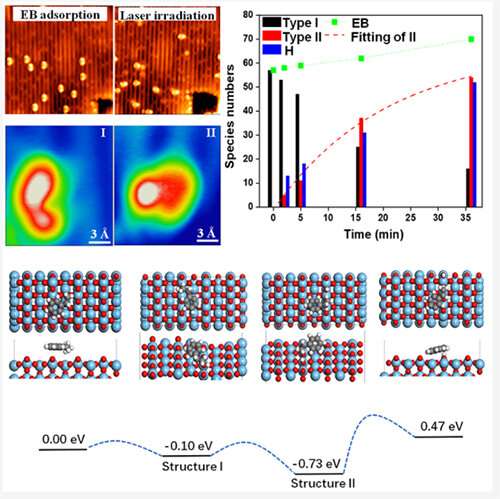
The scientists used the rutile 110 surface of titanium oxide as the model system and the C-H bond molecule ethylbenzene as the model molecule.
They found that ethylbenzene could be induced to remove hydrogen at low temperature (77K) only by photoinduced reaction. They also experimentally observed the images of the reaction steps by in-situ tracking single molecules.
Prof. Pan's team provided support for the theoretical interpretation of the image and confirmed the reaction process.
More information: Haiping Lin et al. In Situ Observation of Stepwise C鈥揌 Bond Scission: Deciphering the Catalytic Selectivity of Ethylbenzene-to-Styrene Conversion on TiO2, The Journal of 萌妹社区ical Chemistry Letters (2020).
Journal information: Journal of 萌妹社区ical Chemistry Letters
Provided by Chinese Academy of Sciences