Illumination technique for cell surface receptors developed by researchers
Human cells sense and communicate via cell surface receptors. Information about the environment is relayed to the inside of the cell through dynamic changes in their arrangement or conformation. To gain better understanding of these dynamics, researchers have developed a variety of imaging methods that allow them to observe receptors in real time.
Birthe Meineke and Johannes Heimg盲rtner have jointly developed a method in Simon Els盲sser's lab at Science For Life Laboratory Stockholm to label and image receptors on live cells with two colors.
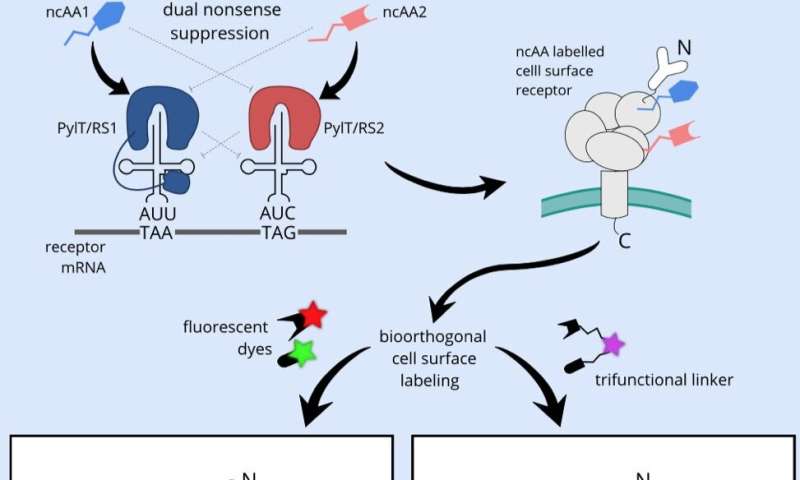
Unlike existing live-cell microscopy methods, which typically rely on sizeable fluorescent proteins fused to the receptor, here, only two amino acids in the receptor of interest are exchanged for noncanonical amino acids. These two synthetic building blocks act as chemical handles, each compatible with a different fluorescent dye, using so-called bioorthogonal chemistry. The method allows the installation of two differently colored dyes on live cells in almost any position of the receptor. Hence, fluorescent assays are possible that not only read out the localization of the receptor, but also report on conformational changes involved in sensing the environment and transducing extracellular signals to the cell interior.
Many receptors, such as the large family of G-protein coupled receptors, are important drug targets. Mechanistic insight into the action of drugs on those receptors has so far predominantly been collected on purified receptors in an in vitro environment. The new method, successfully demonstrated by the team on a G-protein-coupled receptor and a Notch receptor, expands the possibilities to study natural behavior and pharmacology of receptors in their native setting in the membrane of live cells.
The study is published in Cell Reports.
More information: Cell Reports (2020).
Journal information: Cell Reports
Provided by Science For Life Laboratory