Nuclear pore transport puzzle solved by super-resolution microscopy
A study has solved a long-standing debate about the transport of essential proteins, implicated in many human diseases, through one of the cell's most complex and sophisticated structures.
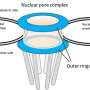
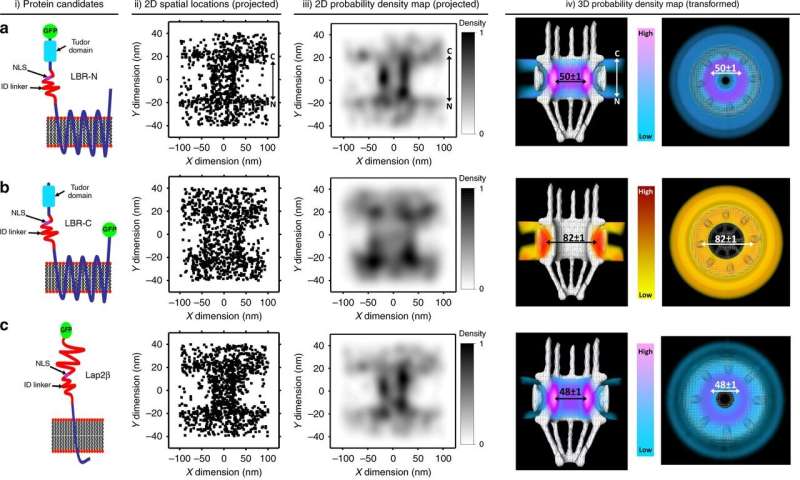
The results reveal that most Nuclear Envelope Transmembrane (NET) proteins travel into the cell's nucleus through small tunnels, known as peripheral channels, whose existence has long been debated by scientists.
Mistakes in the location or function of NET proteins are linked to many human diseases such as muscular dystrophies, cardiomyopathy, blood and bone disorders, and cancers.
Most soluble proteins travel via Nuclear Pore Complexes (NPCs), vast tunnel-like structures spanning the nuclear envelope—the protective membrane that surrounds the nucleus, the command centre of the cell.
"NPCs are one of the cells most important molecular machines, controlling the transport of proteins and RNA into and out of the nucleus, but their size and complexity has led to many different theories emerging on NET protein transport." says a lead author of the study, Professor Eric Schirmer at the University of Edinburgh.
The team, including University of Edinburgh and Temple University (Philadelphia, U.S.) researchers, used a new type of super-resolution microscopy, called single-point edge-excitation sub-diffraction (SPEED) that tracks the movement of individual protein molecules in living cells.
The study is the first to find conclusive functional proof of the existence and importance of smaller peripheral channels, at the edge of central NPC channels, for NET protein transport.
The discovery could explain why some mutated NET proteins end up in the wrong location and cause disease, potentially paving the way to new treatment strategies.
NET proteins help ensure the cell's genetic material, found inside the nucleus, is correctly organised, read and used. To achieve this they must travel to the correct location on the inside of the nuclear envelope.
The team found that blocking peripheral tunnels stopped NET transport, even though the larger Nuclear Pore Complexes were still functional.
Blocking the NPC tunnels did not disrupt transport, although the team found that 10% of NET proteins straddle both tunnels, using them simultaneously for faster and more efficient transport.
"Nobel Laureate Gunter Blobel was a visionary in his suggesting that the NPCs—huge tunnel complexes containing hundreds of structural proteins—were highly plastic and dynamic." says Schirmer. "This is underscored by this study where NETs simultaneous usage of both central and peripheral channels could only happen if the NETs effectively slice through this dynamic protein complex."
The results also suggest that peripheral channels are an important backup mechanism, for example when virus infection blocks the larger NPC tunnels.
The study, published in the journal Nature Communications, was funded by the US National Institutes of Health and Wellcome.
More information: Krishna C. Mudumbi et al. Nucleoplasmic signals promote directed transmembrane protein import simultaneously via multiple channels of nuclear pores, Nature Communications (2020).
Journal information: Nature Communications
Provided by University of Edinburgh